what possible disadvantage could there be to infusing a large volume of an isosmotic nacl solution?
More 90% of hospitalized patients receive I.V. therapy during their hospital stay, typically equally a continuous infusion. Amongst the reasons a patient may receive I.V. therapy are to:
* replace blood or other fluids lost through surgery, trauma, diarrhea, or airsickness
* maintain fluid balance, as when patients are N.P.O. or can't beverage enough fluid for other reasons
* right electrolyte imbalances
* provide a medium for administering medications and nutritional support.
* In this commodity, I'll talk over when various I.V. fluids are advisable, and why. Allow'due south offset by reviewing the principles behind fluid and electrolyte remainder.
Back to basics
Water makes up almost 60% of an developed's body weight and almost lxxx% of a neonate'due south body weight. The corporeality of water normally varies somewhat based on such factors as historic period, sexual activity, and per centum of body fatty.
Most fluid-near 40% of body weight-is in the intracellular compartment, or inside the cells. The balance is in the extracellular compartment, which consists of:
* intravascular fluid (in the blood vessels)
* interstitial fluid (between the blood vessels and cells)
* transcellular fluid (cerebrospinal, pleural, peritoneal, and synovial fluids).
Fluid moves betwixt the fluid compartments by osmosis, a procedure that regulates water and electrolytes so that their distribution and composition in the compartments remain stable. The rate of osmosis depends on the osmotic pressure within the patient's tissues. This pressure draws h2o through semipermeable membranes, such equally a cell membrane.
Responding to osmotic pressure, fluid can move into or out of the cell. The amount of osmotic pressure depends on the ratio between the concentration of ions in the infused solution and the concentration of ions in jail cell fluid. Water moves from an area of low ion concentration (a hypotonic solution) to an expanse of higher ion concentration (a hypertonic solution).
When the number of protein molecules in plasma is low, such equally in proteinuria seen with uncontrolled diabetes or poly peptide-calorie malnutrition known equally kwashiorkor, fluid moves into and stays in the interstitial spaces, where it's unavailable to meet the trunk'due south hydration needs. This is a type of tertiary-space fluid shift, also called third-spacing. This status sequesters fluid in the interstitial and intracellular spaces and in a third-body infinite (such as the intestinal lumen) where it doesn't support circulation.
Taking stock of tonicity
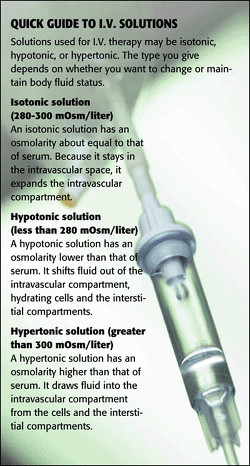
An I.Five. solution's effect on body fluid move depends in part on its tonicity, or the concentration of solutes in solution. Parenteral solutions are classified co-ordinate to their tonicity relative to normal claret plasma. The Infusion Nurses Society (INS) classifies a solution equally isotonic if its tonicity falls within (or near) the normal range for blood serum-between 280 and 300 mOsm/liter. A hypotonic solution has an osmolarity less than 280 mOsm/liter, and a hypertonic solution has an osmolarity greater than 300 mOsm/liter. Hither's how the 3 fluid types deed in the body.
* When an isotonic solution is infused, water neither moves into nor is pulled out of cells because roughly the same concentration of solute is on both sides of the membrane (the tonicity is equivalent). That'south why isotonic solutions such as 0.ix% sodium chloride, Ringer's lactate, Ringer's acetate, and dextrose five% in h2o (D5W), are given to aggrandize circulating volume and replace actual fluid losses. Considering these solutions aggrandize the intravascular compartment, closely monitor the patient for signs and symptoms of fluid overload, especially if he has a history of hypertension or heart failure.Although D5W is isotonic in the bag, information technology acts like a hypotonic solution once information technology enters the bloodstream because simple sugars such equally dextrose are the preferred free energy source for cells. The low concentration of dextrose in D5W is quickly consumed by the cells lining the vein and circulating in the bloodstream. Employ this solution with caution in patients at take a chance for increased intracranial pressure (ICP).The liver converts lactate to bicarbonate, so don't give lactated Ringer'southward solution if the patient has a serum blood pH above 7.five or liver disease-he won't be able to metabolize the lactate, worsening his alkalosis.
* Normally infused hypotonic solutions include 0.45% sodium chloride or 0.25% sodium chloride (with or without D5W). Potassium chloride may be added in depression concentrations to replace losses from the gastrointestinal system. When a hypotonic solution is administered, it puts more h2o in the serum than is found inside cells. Equally a result, water moves into the cells, causing them to swell.Although hypotonic solutions help replace intracellular fluid, the extra water likewise moves into the cells of the tunica intima of the vein at the catheter insertion site. This may cause the cells to cracking and burst, exposing the vein'south basement membrane and potentially leading to phlebitis and infiltration. Sentinel all I.V. sites carefully for signs of phlebitis (erythema at the site with or without pain and edema, palpable venous string, streak formation, and purulent drainage) and infiltration (coolness, swelling, and discomfort).Because hypotonic solutions take the potential to crusade sudden fluid shifts from blood vessels into cells, don't administer them indefinitely. Finish infusing a hypotonic solution in one case the patient can drink enough to see his fluid needs. Failing to practise so could cause cardiovascular collapse from intravascular fluid depletion and increased ICP from fluid shift into brain cells.Don't give hypotonic fluids to patients already at risk for increased ICP, such equally those existence treated for stroke or head trauma and those who've had neurosurgery. Likewise avoid giving hypotonic solutions to patients at risk for tertiary-space fluid shifts, such as those with severe burns, traumatic injuries, or low-serum protein levels from malnutrition or liver affliction.
* When hypertonic fluids are infused, water moves out of the cells in an attempt to dilute the infusate, shrinking the cells. When they shrink at the I.V. infusion site, the basement membrane of the vein's lining is exposed, creating the risk of phlebitis and infiltration every bit described above for hypotonic infusions.
Hypertonic solutions, used to help reestablish equilibrium in electrolyte and acid-base imbalances, include electrolyte replacement solutions and parenteral nutrition solutions. But considering hypertonic solutions can cause astringent damage to the vein, the INS's standards of practise mandate that all fluids with an osmolarity greater than 600 mOsm/liter be infused through a central venous admission device for greater hemodilution. This includes solutions containing more 10% dextrose, 5% poly peptide hydrosylate, and loftier electrolyte concentrations. If you lot're unsure of a solution'due south concluding concentration, check with your pharmacy.
Closely monitor whatsoever patient receiving a hypertonic solution for circulatory overload. Don't requite hypertonic solutions to a patient with whatever status that causes cellular dehydration, such as diabetic ketoacidosis. Nor should any patient with dumb centre or kidney function receive an infusion of hypertonic solution-his system just can't handle the extra fluid.
Kelli Rosenthal is president and master executive officeholder of ResourceNurse Continuing Education, Inc., of Malverne, Due north.Y., and president of the Association for Vascular Access.
 | Figure. No caption available. |
SELECTED REFERENCES
Source: https://www.nursingcenter.com/journalarticle?Article_ID=652137&Journal_ID=54016&Issue_ID=652101
0 Response to "what possible disadvantage could there be to infusing a large volume of an isosmotic nacl solution?"
Post a Comment